
The flow of energy maintains order and life. Entropy is a measure of disorder: cells are not disordered and so have low entropy. In the process of energy transfer, some energy will dissipate as heat.
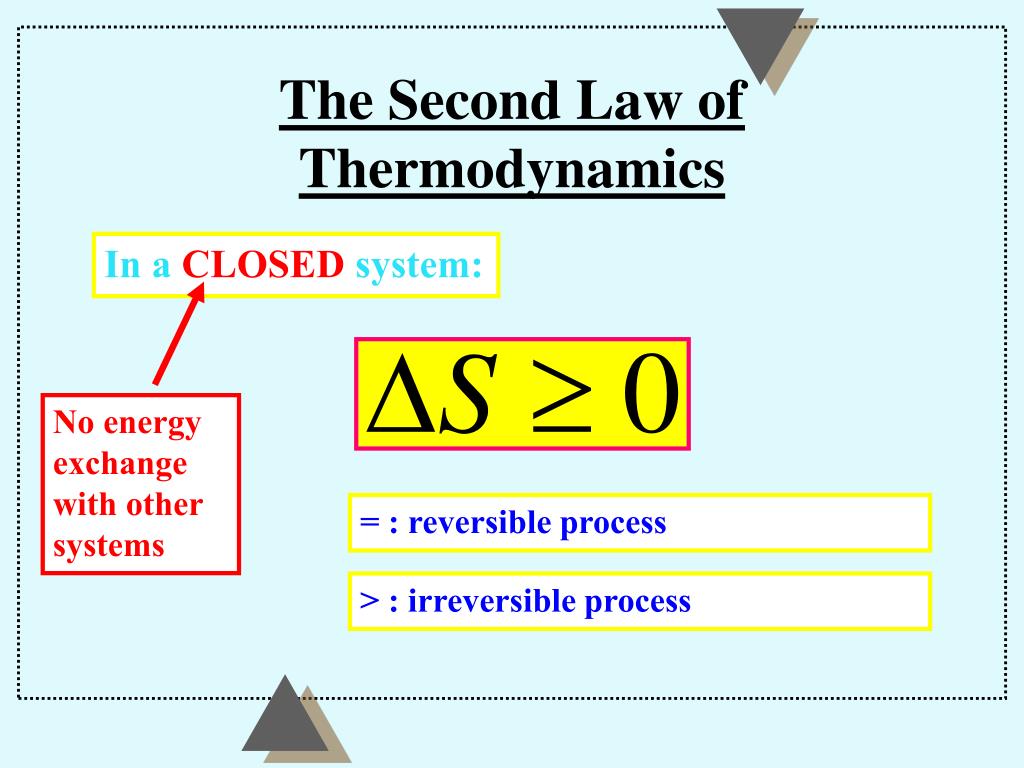
Once the potential energy locked in carbohydrates is converted into kinetic energy (energy in use or motion), the organism will get no more until energy is input again. The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy. In essence, energy can be converted from one form into another. The First Law of Thermodynamics also called the law of conservation states that energy is always conserved it cannot be created or destroyed. The total amount of energy and matter in the Universe remains constant, merely changing from one form to another. The First Law of Thermodynamics states that energy can be changed from one form to another, but it cannot be created or destroyed. In the mid-nineteenth century, this dismal fate came to be known as the ‘cosmic heat death.’” The same slow degeneration afflicts all the stars in the universe. Eventually the sun will run out of fuel and cease to shine. One of the most conspicuous examples is in the way that the sun slowly burns up its nuclear fuel, spewing heat and light irretrievably into the depths of space, and raising the entropy of the cosmos with each liberated photon.


 0 kommentar(er)
0 kommentar(er)
